Antibody binding to pathogen is not sufficient to kill the organism. The Fc portion of immunoglobulin (Ig) can recruit complement or phagocytes to kill the organism. In this way antibodies harness innate effector mechanisms to kill microbes. Antibody protects by
♦Neutralising toxins
♦Neutralising organisms
♦Activating complement
♦Opsonising organisms.
NEUTRALISING TOXINS Toxins bind to receptors on cells, altering cell function. If antibody prevents interaction of toxin with receptor, cell function remains unaffected and the toxin is neutralised. Neutralisation is dependent on the antigen-binding domain only, and is not affected by the Fc portion of Ig (Figure 1.8).
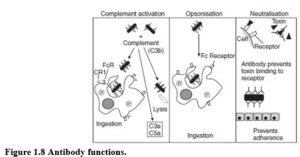
NEUTRALISING ORGANISMS To establish infection organisms must adhere to epithelial surfaces using a limited number of adhesion molecules. Antibodies that bind to key portions of these adhesion molecules prevent organisms binding to mucosae and subsequent infection. Neutralisation of organisms is only affected by the antigen-binding domain, and not by the Fc portion. However, the antibody isotype determines the ability of antibody to cross mucosal surfaces.
COMPLEMENT ACTIVATION IgG and IgM activate complement via the classical pathway. This generates anaphylatoxins (C3a and C5a) that activate mast cells and attract neutrophils to the site of infection.
Deposition of C3 on the organism results in assembly of the membrane attack complex (MAC). In susceptible organisms, insertion of MAC causes leakage of cellular contents and lysis. Bacterial capsules may inhibit MAC assembly, conferring resistance to complement- mediated lysis. Complement activation requires the Fc portion of Ig, and the effectiveness of antibodies at activating complement varies with the isotype.
OPSONISATION Opsonisation is the process whereby microbes and other cells are flagged for phagocytosis. Phagocytes (neutrophils and macrophages) ingest some organisms in the absence of antibody, however, this is relatively inefficient. Antibody-coated organisms are usually phago- cytosed. The antibody acts as a bridge between the organism and Fc receptor on the phagocyte. Binding of Ig to the Fc receptor stimulates phagocytosis and the respiratory burst. Fc receptor activation renders the phagocyte competent to kill most pathogens. Opsonisation requires the Fc portion of the antibody molecule.
Complement (particularly C3b) deposition acts synergistically with antibody to greatly improve opsonisation. C3 fragments bind complement receptors on phagocytes, stimulating particle uptake and activation of neutrophils and macrophages. Co-stimulation of neutrophils through Fc and C3 receptors results in greatly enhanced activation.
ANTIBODY AFFINITY Antibody affinity is the binding strength of an antibody-binding site to an antigen. High affinity results in tight binding and less chance of antibody dissociating from antigen. Different antibody molecules produced in response to the same antigen vary in their tightness of binding. Antibodies produced by a memory response have higher affinity than in a primary response.
ANTIBODY AVIDITY An antibody’s avidity is determined by the combined binding strength of all antigen-binding sites. More binding sites result in increased total binding strength or avidity of antibody binding to antigen.
IMMUNOGLOBULIN ISOTYPE AND FUNCTION
Antibody isotypes have functionally distinct properties, determined by the immunoglobulin constant regions. The constant region recruits cells and molecules to help destroy pathogens. Additionally, each antibody class has different biological activities, capable of dealing with different microbes at different sites (Table 1.2).

IgG IgG is the most abundant antibody and is found in extracellular fluid. IgG crosses the placenta providing passive immunity that persists for 3-6 months after birth. IgG1, IgG2, IgG3 and IgG4 have slightly different sequences in their heavy chains and corresponding differences in their functions.
IgA IgA is the main immunoglobulin in secretions (colostrum, saliva etc.) where it exists as a dimer. IgA is secreted locally by plasma cells in the mammary and salivary glands, and along the respiratory, gastrointestinal and genitourinary tracts. IgA is transported through the epithelial cells into the lumen. Secretory IgA contains a secretory component (SC) and a J-chain (joining chain) required for transepithelial transport and stability of the IgA molecule. IgA is the major component of the adaptive immune response at mucosal surfaces.
IgM IgM is the first antibody produced by B cells. It is expressed on B cells as the B cell antigen receptor. Pentameric secreted IgM is also found in blood. IgM antibodies usually have low affinity antigen-binding sites, however, since IgM has 10 antigen binding sites per molecule, the overall binding avidity is high. This pentameric structure makes it very effective for activating the complement system.
IgD IgD is present in low quantities in the circulation. The principal known function is as an antigen receptor on B cells. B cells can express both IgM and IgD, which have the same antigen specificity.
IgE IgE is present in the serum in nanogram quantities. IgE plays an important role in protection from infection by parasites and in immediate hypersensitivity responses.




