Antibodies, also called immunoglobulins (Ig), are glycoproteins, produced by plasma cells, that bind antigens with a relatively high specificity and affinity.
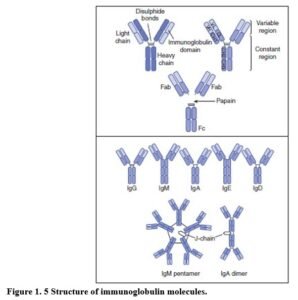
ANTIBODY STRUCTURE Antibody molecules are comprised of two identical light chains and two identical heavy chains. Light chains may be kappa (K) or lambda (λ) in type, but this does not affect antibody function.
The antibody isotype or class is determined by the heavy chain (μ, γ, α, ε or δ) giving rise to immunoglobulin M (IgM), immunoglobulin G (IgG), immunoglobulin A (IgA), immunoglobulin E (IgE) and immunoglobulin D (IgD), respectively. In addition to differences in the basic antibody unit, antibody isotypes differ in the number of units in a typical molecule – IgM is usually pentameric, IgA dimeric and both IgG and IgE monomeric (Figure 1.5). The five isotypes differ greatly in functional activity. IgG is subdivided into the subclasses IgG1, IgG2, IgG3 and IgG4 and IgA into IgA1 and IgA2 subclasses. Antibody subclasses differ in heavy chain amino acid sequences conferring functional differences.
Variable and constant regions of heavy and light chains Heavy and light chains have a variable region (composed of variable domains), and a constant region (composed of constant domains). Variable regions of heavy and light chains generate two identical antigen-binding sites, conferring specificity on the antibody. The heavy-chain constant region determines the antibody isotype and functional properties.
Functionally distinct fragments of the antibody molecule – Fc and Fab
Proteolytic cleavage by the enzyme papain cleaves immunoglobulin into two Fab fragments and one intact Fc fragment. The Fab fragment binds antigen and the Fc fragment contains constant domains.
ALLOTYPES Non-functional polymorphisms occur in genes encoding heavy chain constant regions giving rise to allotypes. Allotypes are differences between individuals’ antibody molecules, comparable to different blood groups. There is no functional difference between antibodies of different allotypes.
IDIOTYPES Idiotypes are unique antigenic determinants found in antigen-binding sites of antibodies. They result from different amino acid sequences in antigen-binding sites that determine the antibody specificity. Idiotypes are unique to antibodies produced by the same clone of B cells.
GENERATION OF DIVERSITY The antibody response to an antigen is diverse, generating many different B cell clones each with its own unique specificity. An individual’s collection of antibody specificities is called
the antibody repertoire. Antibody diversity is generated during B cell development by random combination of gene segments from heavy-chain and light-chain gene groups (Figure 1.6).
There are three gene clusters encoding immunoglobulins:
♦κ-chain clusters are found on chromosome 2
♦λ-chain genes are found on chromosome 22
♦Heavy-chain gene clusters are found on chromosome 14.
Variable and constant regions of immunoglobulin molecules are encoded by V gene segments and C gene segments, respectively.
Somatic recombination generates variable regions The variable region of heavy and light chains is generated from separate V region gene segments by a process of DNA rearrangement known as somatic recombination. The heavy-chain V region gene segments include Variable (VH), Diversity (DH) and Joining (JH), while the light chain contains variable (VL) and joining segments (JL).
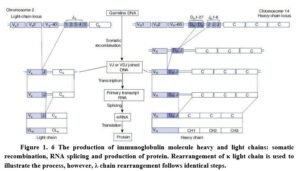
V region recombination involves the ‘VDJ recombinase’ enzyme complex, which includes generic DNA cleavage and repair enzymes and lymphocyte-specific components. RAG-1 and RAG-2 are lymphocyte-specific enzymes, essential for VDJ recombination. VDJ recombination results in removal of intervening gene segments, permanently changing the genomic DNA in the B cell. The assembled variable regions of both heavy and light chains join to their respective constant regions after transcription, by RNA splicing. Heavy- chain C region genes have several gene segments, each encoding the constant region for a different antibody class (Cg, Cy, Ca, C8, Ce). κ chains have one C gene segment for its constant region, while λ chains have four C gene segments. Heavy chain genes undergo rearrangement before light chain genes.
Regulation of immunoglobulin gene rearrangement ensures that each B cell only expresses one rearranged heavy chain and one rearranged light chain. Therefore, each B cell expresses millions of identical surface antibodies.
ANTIBODY DIVERSITY IS GENERATED BY FOUR PROCESSES, WHICH TOGETHER CREATE A VAST REPERTOIRE OF ANTIBODY SPECIFICITIES FROM A LIMITED NUMBER OF GENES
1 There are several different gene segments making up the variable regions of both heavy and light chains (Table 1. 1). Different combinations of variable region gene segments recombining randomly generate considerable diversity in the antigen-binding site. This is known as combinatorial diversity.
2 Pairing of different heavy and light chains increases diversity.
3 During recombination, imprecise joining of gene segments results in insertion of additional nucleotides – known as junctional diversity.
4 Somatic hypermutation introduces point mutations into variable regions of rearranged heavy and light chain genes. This allows the antibody specificity to be changed after recombination occurs. Mutant antibody molecules may bind antigen better or less well than the original antibody. B cells expressing higher affinity antibody are selected and mature into antibody-secreting cells. Somatic hypermutation and selection of high affinity antibody gives rise to the physiological phenomenon of affinity maturation, whereby the affinity of antibodies produced improves as an immune response develops.

Rearrangement of gene segments occurs in developing B cells in bone marrow. Somatic hypermutation occurs in B cells in secondary lymphoid organs after functional antibody has been expressed.
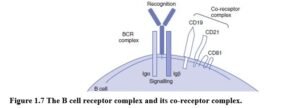
THE B CELL RECEPTOR COMPLEX Immunoglobulin was first discovered in plasma. However, before antibody can be secreted, immunoglobulin must function as the cell-surface antigen receptor, that is, the B cell receptor (BCR). Antigen binding to BCR activates B cells, leading to clonal expansion and differentiation into antibody-secreting plasma cells. B cells initially express transmembrane IgM and when activated differentiate into plasma cells. B cell activation usually requires specialised interactions with helper T cells that provide additional signals.
The BCR complex includes the antigen receptor, surface immunoglobulin, associated with two other polypeptides, Iga and Igp. When sIg is crosslinked, these proteins transmit signals leading to B cell proliferation and differentiation. They are also required for the expression and assembly of immunoglobulin (Figure 1.7). B cell co-receptors (CD21, CD19 and CD81) play an important role in enhancing or inhibiting B cell activation.