Atoms tend to arrange themselves in the most stable patterns possible, which means that they have a tendency to complete or fill their outermost electron orbits. They join with other atoms to do just that. The force that holds atoms together in collections known as molecules is referred to as a chemical bond. There are two main types and some secondary types of chemical bonds:
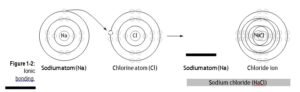
v Ionic bond: This chemical bond (shown in Figure 1-2) involves a transfer of an elec- tron, so one atom gains an electron while one atom loses an electron. One of the resulting ions carries a negative charge, and the other ion carries a positive charge. Because opposite charges attract, the atoms bond together to form a molecule.
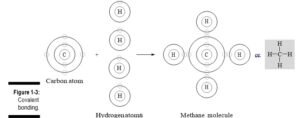
v Covalent bond: The most common bond in organic molecules, a covalent bond (shown in Figure 1-3) involves the sharing of electrons between two atoms. The pair of shared electrons forms a new orbit that extends around the nuclei of both atoms, producing a molecule. There are two secondary types of covalent bonds that are relevant to biology:
- Polar bond: Two atoms connected by a covalent bond may exert different attractions for the electrons in the bond, producing an unevenly distrib- uted char The result is known as a polar bond, an intermediate case between ionic and covalent bonding, with one end of the molecule slightly negatively charged and the other end slightly positively charged. Although the resulting molecule is neutral, at close distances the uneven charge dis- tribution can be important. Water is an example of a polar molecule; the oxygen end has a slight positive charge whereas the hydrogen ends areslightly negative. Polarity explains why some substances dissolve readily in water and others do not.
- Hydrogen bond: Because they’re polarized, two adjacent H2O (water) mole- cules can form a linkage known as a hydrogen bond, where a (electronega- tive) hydrogen atom of one H2O molecule is electrostatically attracted to the (electropositive) oxygen atom of an adjacent water Consequently, molecules of water join together transiently in a hydrogen-bonded lattice. Hydrogen bonds have only about 1⁄20 the strength of a covalent bond, yet even this force is sufficient to affect the structure of water, producing many of its unique properties, such as high surface tension, specific heat, and heat of vaporization. Hydrogen bonds are important in many life processes, such as in replication and defining the shape of DNA molecules.
A chemical reaction is the result of a process that changes the number, the types, or the arrangement of atoms within a molecule. The substances that go through this process are called the reactants. The substances produced by the reaction are called the products.
Chemical reactions are written in the form of an equation, with an arrow indicating the direction of the reaction. For instance: A + B ® AB. This equation translates to: Atom, ion, or molecule A plus atom, ion, or molecule B yields molecule AB.
When elements combine through chemical reactions, they form compounds. When compounds contain carbon, they’re called organic compounds. The four families of organic compounds with important biological functions are
v Carbohydrates: These molecules consist of carbon, hydrogen, and oxygen in a ratio of roughly 1:2:1. If a test question involves identifying a compound as a car- bohydrate, count the atoms and see if they fit that ratio. Carbohydrates are formed by the chemical reaction process of concentration, or dehydration synthe- sis, and broken apart by hydrolysis, the cleavage of a chemical by a reaction that adds water. There are several subcategories of carbohydrates:
- Monosaccharides, also called monomers or simple sugars, are the building blocks of larger carbohydrate molecules and are a source of stored energy (see Figure 1-4). Key monomers include glucose (also known as blood sugar), fructose, and These three have the same numbers of carbon (6), hydrogen (12), and oxygen (6) atoms in each molecule — for- mally written as C6H12O6— but the bonding arrangements are different. Molecules with this kind of relationship are called isomers.
- Disaccharides, or dimers, are sugars formed by the bonding of two mono- saccharides, including sucrose (table sugar), lactose, and
- Polysaccharides, or polymers, are formed when many monomers bond into long, chain-like Glycogen is the primary polymer in the body; it breaks down to form glucose, an immediate source of energy for cells.
v Lipids: Commonly known as fats, these molecules contain carbon, hydrogen, and oxygen, and sometimes nitrogen and phosphorous. Insoluble in water because they contain a preponderance of nonpolar bonds, lipid molecules have six times more stored energy than carbohydrate molecules. Upon hydrolysis, however, fats form glycerol and fatty acids. A fatty acid is a long, straight chain of carbon atoms with hydrogen atoms attached (see Figure 1-5). If the carbon chain has its full number of hydrogen atoms, the fatty acid is saturated (examples include butter and lard). If the carbon chain has less than its full number of hydrogen atoms, the fatty acid is unsaturated (examples include margarine and vegetable oils). All fatty acids contain a carboxyl or acid group, –COOH, at the end of the carbon chain.
Phospholipids, as the name suggests, contain phosphorus and often nitrogen and form a layer in the cell membrane. Steroids are fat-soluble compounds such as vitamins A or D and hormones that often serve to regulate metabolic processes.

v Proteins: Among the largest molecules, proteins can reach molecular weights of some 40 million atomic units. Proteins always contain the four HONC elements — hydrogen, oxygen, nitrogen, and carbon — and sometimes contain phosphorus and sulfur. The human body builds protein molecules using 20 different kinds of smaller molecules called amino acids (see Figure 1-6). Each amino acid molecule is composed of an amino group, –NH2, and a carboxyl group, –COOH, with a carbon chain between them. Amino acids link together by peptide bonds to form long molecules called polypeptides, which then assemble into proteins. Examples of proteins in the body include antibodies, hemoglobin (the red pigment in red blood cells), and enzymes (catalysts that accelerate reactions in the body).

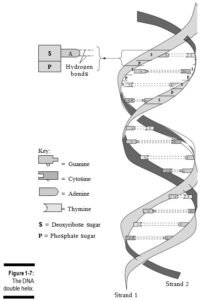
v Nucleic acids: These long molecules, found primarily in the cell’s nucleus, act as the body’s genetic blueprint. They’re comprised of smaller building blocks called nucleotides. Each nucleotide, in turn, is composed of a five-carbon sugar (deoxyri- bose or ribose), a phosphate group, and a nitrogenous base. The nitrogenous bases in DNA (deoxyribonucleic acid) are adenine, thymine, cytosine, and gua- nine; they always pair off A-T and C-G. In RNA (ribonucleic acid), which occurs in a single strand, thymine is replaced by uracil, so the nucleotides pair off A-U and C-G. In 1953, James Watson and Francis Crick published their discovery of the three-dimensional structure of DNA — a polymer that looks like a ladder twisted into a coil. They called this structure the double-stranded helix (see Figure 1-7).